CAR T Cell Therapy Bio

My name is Nicole Gularte and I am a two-time CAR T cell therapy recipient. I first became involved with CAR T therapy in 2014 after having my cells collected by the research team at the University of Pennsylvania’s Abramson Cancer Center. With this particular therapy still being in it’s infancy stage, many were unsure about the safety and efficacy of this new form of immunotherapy. After learning about the story of Emily Whitehead – the first child in the world to receive the treatment – I was inspired to fight for my place in the new clinical trial at Penn. I qualified for the trial and received my modified CD19 CAR T cells in September 2016. Just 28 days later, I was declared cancer free.
I was first diagnosed with acute lymphoblastic leukemia (ALL) in 2010 and am currently on my 8th relapse. In 2018, after my two-year post CD19 CAR T therapy checkup, doctors at Penn informed me of a relapse. Lucky for me, the relapse came just in time for a new CAR T clinical trial at Penn. I would be the first patient in this new CD22 targeted clinical trial. I entered the trial and after 28 days, I became cancer free once again.
In April 2019, I was informed of my most recent relapse. This summer, I will be one of the first patients to participate in a dual CAR T clinical trial at Penn. In this trial there are two receptors that will be targeted – both CD19 and CD22.
You can follow this latest CAR T journey by visiting my blog page.
In an effort to help raise awareness for cell therapy research, and to help others navigate and access cell therapy clinical trials, I work with the Emily Whitehead Foundation as well as other patients and key stakeholders who are dedicated to making a difference in the lives of cancer patients and families.
For information or assistance, please contact me at:
Cell Phone: 925-436-0010
Email: inspiredhero@outlook.com
It All Started With A Girl Named Emily Whitehead

Watch News Video About Nicole’s CAR T journey:
Click here for news video
From Hospice to Cured

My Story Begins Here and Ends with A Cure
Thanks to Dr. Carl June and his team – along with many other teams in this world – I am a seven-time leukemia Survivor. When I reflect back on the date of my initial diagnosis (October 26, 2010), I had no clue as to the events that would transpire in the years to come. I certainly would not have predicted a journey so intense and chaotic, and yet with such an extraordinary outcome.
The last seven years has been a long battle; a quest full of challenge, risk, self advocacy, and research. Over the course of six years (2010 – 2016), I have endured six different relapses (three bone marrow relapses, two CNS relapses, and one isolated CNS relapse to both eyes). During this period of uncertainty and opportunity (some call it “brave”, I call it being “fortunate”), I was able to participate in three vastly different clinical trials. Each clinical trial ultimately saved my life – one having acted as a bridge to another – and all three have been approved by the FDA to treat my specific leukemia – Acute Lymphoblastic Leukemia (ALL). The following are details on these three clinical trials:
- CALBG Study – My first trial (at Stanford Cancer Center, Oct 2010 – Apr 2014), is used as a primary method of treatment for this adolescent form of leukemia and consists primarily of chemotherapy and radiation. This standard protocol is not only highly toxic, but very lengthy. With a minimum of two years (for girls) and three years (for boys), both children and adults will spend the majority of these years in the hospital or daily trips to an infusion treatment area (ITA). In addition, the side effects are daunting, frightening and unfathomable to most. Although remission rates are high for adolescents (80% +), these rates are far less in adults with this childhood form of leukemia (~45%). It is important to consider and understand; however, the long-term side effects that all patients may be faced with with this form of treatment.
- Inotuzimab Ozogamicin – My second trial (also at Stanford Cancer Center, May 2014 – Jun 2014), was a Phase III targeted antibody. This earlier form of immunotherapy acts differently from chemotherapy in that it primarily kills cancer cells rather than killing both cancerous and healthy cells in the body. In my particular case, this therapy acted as a “bridge” to a more advanced and new form of therapy – CAR T-cell therapy.
- CAR T-cell or T-cell Therapy – In July 2016 I was on hospice with only three to five weeks left to live. After getting baptized and making funeral arrangements, test results showed I qualified for a new clinical trial at UPenn and 28 days later I became a seven-time leukemia survivor. The journey to my getting CAR T-cell therapy at UPenn, however; was not so smooth. I began fighting for CAR T-Cell treatment in 2014 and it took over two years and half-a-dozen “no’s” before I was treated. It is ironic that on the anniversary date of my original diagnosis, I shared my story at The University of Pennsylvania for the launch of The Parker Institute for Cancer Immunotherapy. Today, I am an active patient advocate and keynote speaker. My partner, Anna Gutkina (also an immunotherapy survivor), and I are working together to support clinical research and building lasting relationships within our communities.
Photos – Journey To Survivorship:
Background
In my first clinical trial at Stanford in 2010 (the CALBG protocol with standard chemotherapy), I spent eight to fourteen hours a day and nearly each day of the week inside of an infusion treatment area (ITA). For two years, I suffered nearly every side effect possible which made life awful. Hospital admissions were far too often and way too long. The treatment was highly toxic and I struggled in nearly every aspect. To illustrate a bit, I have had over 300 lumbar punctures (spinal taps/needle in the spine), over 50 bone marrow aspirates (a procedure where a drill or similar tool is used to collect bone marrow from the back of your hip), and I have spent over 600 nights in various hospitals. All of my procedures have been done while conscious with only a local anesthetic (fortunately, infants and young children are not required to be conscious for both the LP and BM aspirates).
Photos From 1st Clinical Trial at Stanford (Oct 2010 to April 2014):
After an intense two years, I slowly worked my way back into normalcy. But in the middle of recovery, both of my knees went out. Doctors confirmed avascular necrosis (bone death) in both knees, and I was in surgery the following Monday. The avascular necrosis was a side effect from Prednisone, a potent steroid given during treatment. In July 2013, a subchondroplasty (a major procedure for bad joints), was performed on both knees. It took over four weeks just to stand up and put weight on my feet again, and it took eight additional weeks to learn how to walk. Since the outdoors and hiking are my passion, I was torn by the fact that I would never be able to hike again. The emotional recovery from this set back, would take years.
Stay Patient and Trust Your Journey
In April 2014, I was devastated after Stanford doctors delivered news of my cancer relapse. The thought of going through a transplant (my only option at this point in time), had me scrambling for something new. I understood the fact that there was about a 50% chance that I would relapse within five years on the CALBG trial, but I had no idea what a relapse would require – intensive chemotherapy all over again, followed by a bone marrow transplant (if a donor was found). Knowing that a bone marrow transplant has about a 30% success rate and with life-long side effects, I hoped for the best and planned for the worst. When I asked if there were other treatment options, my doctors discussed CAR T-cell, but when I asked what CAR T-cell therapy was, my doctor said, “Nicole, you will never live to see CAR T-cell. Not in your lifetime”. Unsure of what to do with my only option, I quickly checked out of the hospital and went home to conduct my own research. Little did I know how important this decision was to my future survival.
First Relapse – April 2014:
Immunotherapy
A different hematologist from Stanford called me shortly after leaving the hospital and asked if I would come in to discuss the possibility of a new clinical trial. Before I knew it, I was in a Phase III trial for Pfizer’s, Inotuzumab Ozogamicin. This particular drug uses antibodies that target and kill specific cancer cells. Although this new form of immunotherapy could offor a quick remission (without the horrible toxicity and lengthy course of traditional therapy), I understood – and my doctors reiterated – it would not be a lasting remission and the cancer would soon come back without a bone marrow transplant. I was given the drug in the hospital where doctors monitored me for several weeks. Excitingly enough, I achieved a complete remission and was able to return home where I would wait for a transplant donor match. I was beyond grateful and shocked (as my doctors were too) that I achieved a remission without enduring the daunting experience of combination chemotherapy – and it all happened without losing my hair and nails! In fact, this regimen was so much more pleasant, I was able to order an elliptical and have it delivered to my hospital room – with hospital permission, of course (which took a lot of time and effort arguing and debating with a team of doctors over “safety” issues). My efforts soon paid off and the next time my Stanford team of doctors came in during rounds, they found me blissfully gliding away on the elliptical, listening to music while playing Candy Crush Saga on my iPhone. With smiles on their faces and eyes tearing up, one physician said (and the others agreed), “we have worked our entire careers for something like this to happen and it’s happening right now – right in front of our eyes”. It was a magical moment, to say the least.
Myself & Dr. Michaela Liedtke, MD, (New Hematologist and PI for Second Clinical Trial at Stanford), May 2014:
CAR T-Cell Therapy
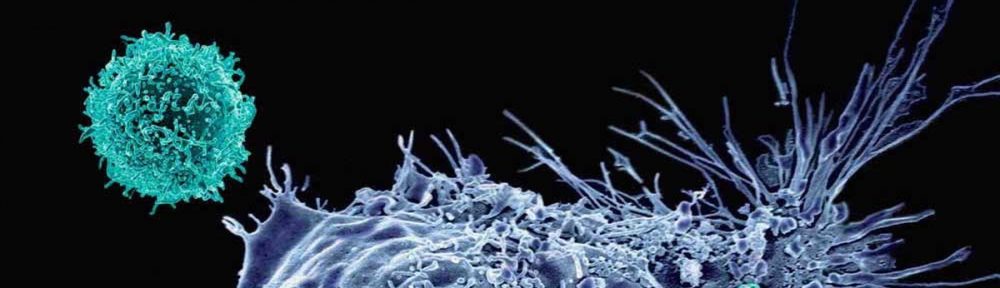
Chimeric Antigen Receptor (CAR) T-cell is a form of immunotherapy where human T-cells are modified, grown in a laboratory, and re-infused back into a patient. The idea of CAR technology, is to have a “receptor” that allows the T-cells to continuously recognize, attack, and kill cancer. Researchers at The University of Pennsylvania opened its first ALL trial for pediatric patients in 2012. Emily Whitehead, just five years-old at the time, had her leukemia return three times. She was the first child in the world to receive T-cell therapy. She is now six years cancer free.
The Butterfly Counts Not Months But Moments and Has Time Enough
I requested Stanford send me to UPenn to discuss the trial, but my team of doctors (and 99% of Stanford’s staff) did not agree that CAR T-cell would be best for me. In fact, most thought and many voiced that I was “crazy” for considering such a new and unpredictable treatment – after all, without a stem cell or bone marrow transplant, my cancer would always return… and given transplant has been the only chance of survival over the past four decades, no doctor was inclined to “back me up”. Should I try to fight for CAR T therapy? Will I have to do this on my own? When reflecting back on the three years of agony from a plethora of chemotherapy cocktails, I was reminded of the daunting list of side-effects that continues to grow and haunt me today. A transplant has serious complications; again, with only a 30% success rate — whereas the CAR T-cell treatment had a 94% success rate. I realized my quality of life was worth more than time that cannot be well spent. From this point forward, I chose and fought for quality, not quantity, for the years ahead of me.
The Road Less Traveled
I took it upon myself, and reached out to UPenn requesting a consultation for their new CAR T adult ALL study. It took a lot of effort and half a dozen “no’s,” before I was able to be heard. In August 2014, with my mom and aunt by my side, I flew out and met the team of hematologists, researchers, nurses, and pathologists. They were unsure about whether I was making an “educated” or “informed” decision, but once I explained my story along with the level of research, they were confident that I was well informed. Dr. Don Siegel, MD, PhD, Director of Pathology and Laboratory Medicine was shocked when he saw me sitting at the apheresis machine – shocked that I had come all the way out from Stanford. He asked how I heard about CAR T-cell therapy and proceeded to show me a video about Emily Whitehead. This was the first time I had learned about Emily and from that moment forward, she became my hero. While collecting my t-cells, Dr. Siegel shared how Dr. Carl June, and Dr. David Porter discovered CAR T-cell therapy. I could sense the passion and determination in his voice. Dr. Siegel then gave me a tour of the new facility (funded by Novartis and later, the Parker Institute for Cancer Immunotherapy), showed me where my t-cells would be stored, and let me take a peek at the laboratory where t-cells would be manufactured. The entire process is phenomenal. After meeting the team, seeing the facility, and hearing the stories behind their discovery, I left UPenn feeling, believing, and knowing that this was the beginning of something amazing, and I wanted to be a part of it. After returning to Stanford, I turned down my transplant donor and explained that I was going to allow myself to relapse just so I could qualify for the CAR T-cell trial – even though (at the time – in 2014), the FDA had a hold on the trial and was not allowing anyone to enter. Not knowing if or when the trial would resume, this was a HUGE risk, but a risk I felt I should take and a risk – I would later find – that would save my life.
First Visit to The University of Pennsylvania, August 2014:
Be the Change You Wish to See in the World
By the end of 2014, I showed no signs of a leukemia relapse. Summer passed. Fall came. Fall went. In November 2014, I decided to move forward with a second bi-lateral knee surgery, because the first surgery did not work and I had difficulty walking. Although it was not as intense as the first surgery, it took years to physically and mentally heal.
Second Bilateral Knee Surgery, October 2014:
In March 2015, I learned that CAR T-Cell trials were opening for pancreatic cancer. The only other young adult I’ve ever known to have cancer, was a good friend, father, and fighter. Jay Cook and I had been friends since my sophomore year of college. He brought a large box full of DVD’s and a Blue Ray player to my hospital room at Stanford when I was first diagnosed in 2010. Three years later he was diagnosed with pancreatic cancer; and it was I, visiting him at the hospital. Just one year later (April 2014), when my leukemia relapsed, Jay passed. He fought a tough battle; I had to keep the fight for him.
Hero, Jay Cook, Passed in 2014 From Stage IV Pancreatic Cancer:
Where There’s A Will There’s A Way
In May 2015, after recovering from Pneumonia, surgery on a detached retina confirmed Leukemia in my eyes. In fact, I had never really achieved remission from the Inotuzumab, rather the leukemia had gone straight to my eyes — an isolated CNS relapse. At this point, I still had not relapsed in the bone marrow which meant I did not qualify for the CAR T study or any other clinical trials. So, I began my third attempt to kick this cancer. I started radiation therapy in both eyes which resulted in total vision loss in the left eye. Shortly after completing radiation, tests verified leukemia had gone to my spinal fluid. I received three different chemotherapy drugs via spinal tap, three days a week, tailored down monthly. Theses treatments continued for over a year. Although the odds were not in my favor, I lived as though they were. My local coordinator at the Leukemia and Lymphoma Society asked if I would share my story at a couple of events, and I felt honored; for it would be the first time I ever shared my story publicly.
Isolated CNS Relapse, Surgery, and Radiation Therapy to Both Eyes (May 2015):
Teamwork Makes the Dreamwork
We are all touched by cancer in some way; if not by now, then somehow, someday, some way. Immunotherapy and CAR T-cell therapy had been granted “breakthrough” cancer treatments for leukemia and was being used in clinical studies for various forms of cancer. As I continued through treatments, I felt the need to raise awareness for these new forms of therapy. I reached out to those wanting to help spread awareness and we created the dream team under the Leukemia & Lymphoma Society, called Team Nicole For Awareness. We raised over 25K for the LLS while I shared my story in various cities and at different events. It was difficult (as a team captain) to manage a team, fundraising events, and give speeches all while receiving treatment. I became overwhelmed and frustrated, but the dream team came through and we all made it happen. My team reminded me that when I fall, I need to get back up.
I am Aware That I am Rare
I completed radiation to both eyes in July 2015, but shortly after, relapsed in the spine once again. I continued to fight my fifth leukemia relapse for the remainder of the year. A full remission was achieved; however, I knew it wouldn’t be lasting.
It was not until April 2016 when Stanford doctors confirmed a bone marrow relapse. I immediately flew out to Philadelphia to take necessary exams and sign all required documentation for the T-cell manufacturing process. I flew back home to California, and had three weeks to pack bags and make arrangements for my six-week stay at U Penn for the T-cell therapy trial beginning in July. During this period, Stanford doctors confirmed my worst nightmare — my most recent spinal test result confirmed yet another relapse in the CNS. This immediately disqualified me from the T-cell trial.
As July approached, I was terribly saddened by the fact that I should have been starting my T-cell therapy trial. Instead, I ended up in the hospital for two weeks with fevers, infections, and severe migraines. The source of the infection was never found, and my migraines were likely a side effect from having over 300 spinal taps. On top of this, I now had leukemia in my lymph nodes. I began to quickly lose hope from this moment forward.
In August, after no improvement, I decided it was time to stop treatment all together. The chemotherapy was toxic and my health was declining. A Stanford palliative care team sent me over to hospice, and hospice sent me home with three to five weeks to live.
Reaching Out
In an effort o obtain guidance from an expert in patient advocacy, I reached out to Thomas Whitehead, father of Emily. I knew Tom and Kari Whitehead would have a great deal of knowledge and experience in this area. After explaining my situation to Tom, he did everything in his power to help me. He even tried to get me my cells under “compassionate care” which would allow me to receive my T-cells at Stanford rather than The University of Pennsylvania. This was the first time ever, that I received help or assistance in my fight against cancer. Tom advocated on my behalf, stood up for my rights, and encouraged me to reach out to parents of others who once faced similar situations.
The Power of Prayer
As I began planning my burial, I realized that although my entire family is Catholic, I had never been baptized. So, my best friend planned a baptism, and a lovely Catholic ceremony marked the most beautiful day in my life. After four weeks of short travel trips and spending time in various Catholic Churches, oddly, I felt very well considering I was due to die any day. After one of our regular talks, Tom Whitehead urged me to go back to Stanford for a blood test. Surprisingly, my labs looked very normal considering I had not had any blood or chemotherapy in over a month. Tom Whitehead and my hematologist talked me into getting one final spinal tap to check the cancer in my CNS. The results revealed a miracle — no cancer detectable. I suddenly qualified for the T-cell trial at U Penn.
Fighting Fire with Fire
On August 28, 2016 – within a week of receiving the good news – I was on a flight to Philadelphia. On September 6th, I received my modified T-cells. Supporters documented my T-cell journey on social media on a regular basis. You can find my Facebook group, Fighting Fire with Fire – Nicole’s Journey U Penn, to learn more. This group is now open to the public. You can also find me on Instagram as Inspiredheroes.

Facebook:
https://www.facebook.com/groups/167695120304168/
Instagram:
https://www.instagram.com/inspiredhero
Twitter:
@fight4cures
LinkedIn:
http://linkedin.com/in/fight4cures
Website:
https://inspiredheroes.org
Paying It Forward
On October 4th I finished my T-cell therapy and achieved a full remission. My journey has made me who I am today. In spite of all my struggles, I am a happier, healthier individual dedicated living a life of purpose. I dedicate much of my time to helping others with all forms of cancer. I have encouraged others to reach out to me for assistance and inspiration.
On October 25th, I shared my story with Dr. Carl June and his research team at the grand opening of The Parker Institute of Cancer Immunotherapy at the University of Pennsylvania. This was a celebration to honor Sean Parker’s $250,000,000 allocation to cancer research. This event also marked the opening of several new clinical trials in the U.S. for solid tumors.

Sean Parker, Nicole Gularte, and Bruce Levine, PhD, at the launch of the Parker Institute for Cancer Immunotherapy at the University of Pennsylvania, Abramson Cancer Center, October 25, 2016